Clinical Teams
What is a teletrial?
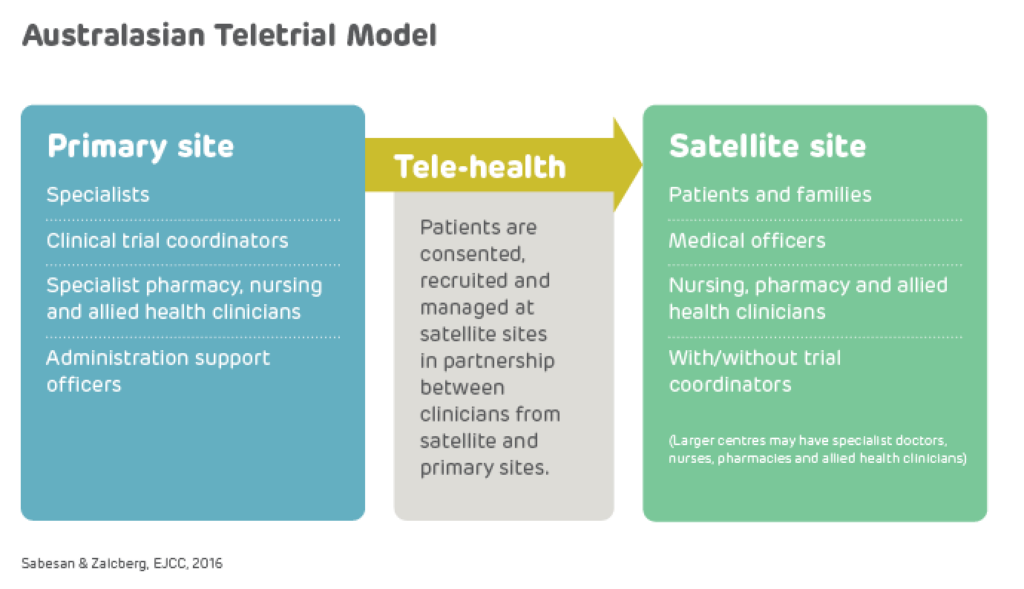
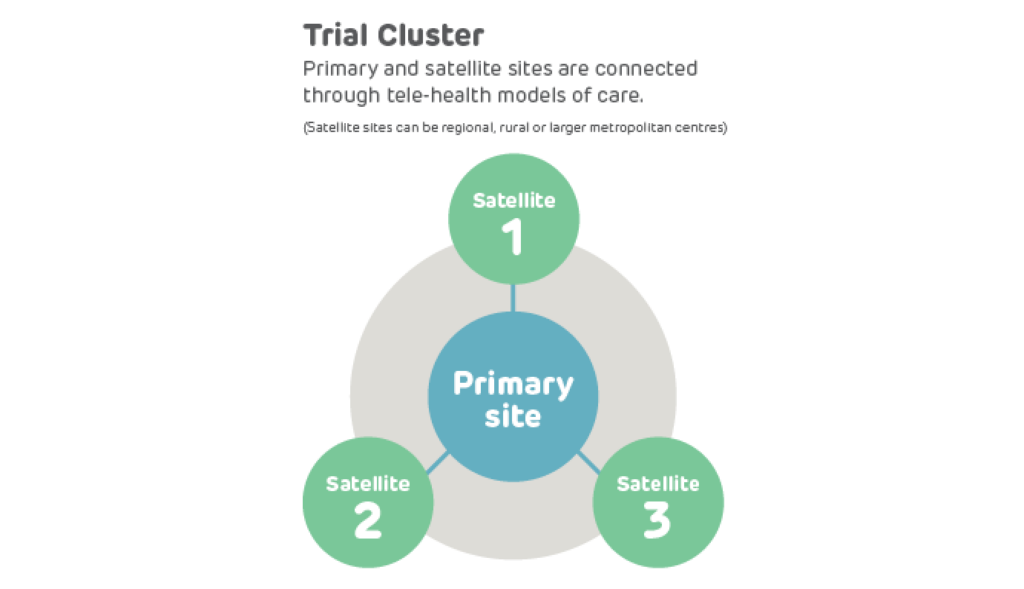
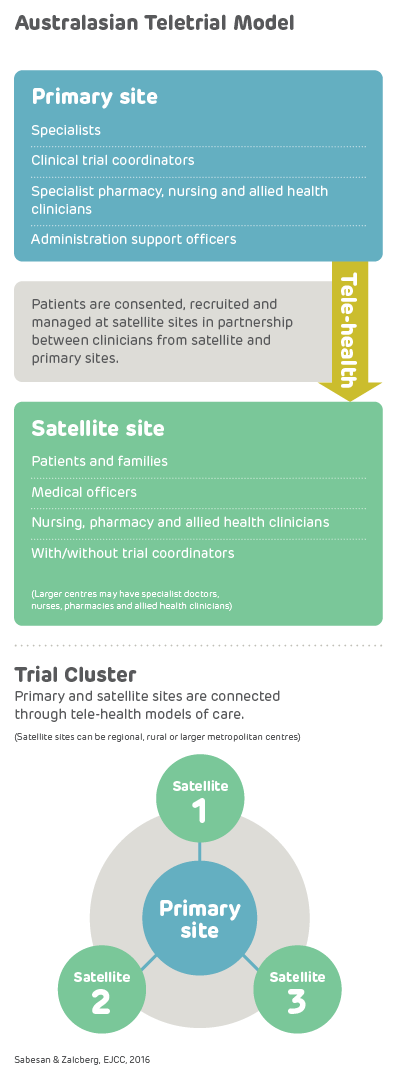
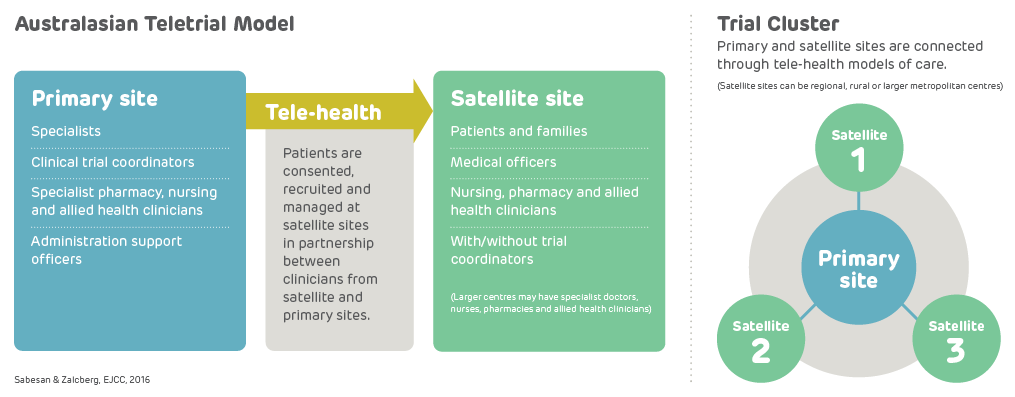
A teletrial is a group of clinical trial sites that work together to conduct a clinical trial under the supervision of a Primary Site.
A teletrial uses telehealth technology to communicate between the Primary Site and Satellite Site/s and enable delivery of aspects of a clinical trial closer to home for patients particularly in regional, rural and remote locations. A Principal Investigator supervises Associate Investigator/s to conduct a clinical trial at a Satellite Site which is geographically remote from the Principal Investigator’s Primary Site.
The Principal Investigator remains responsible for the trial. A detailed Supervision Plan is required, in addition to a Delegation Log required by ICH GCP for all Satellite Sites regardless of experience. Trial participants may have trial visits at both the Primary and Satellite Sites, as determined by the Protocol and Supervision Plan.
The conduct of the trial is detailed under the ‘head agreement’ (Clinical Trial Research Agreement/Clinical Trial Agreement) between the Sponsor and the Principal Investigator’s Institution and a Sub-Contract between the Primary Site and the Satellite Site Institutions (see Clinical Trial Research Agreement – Tele Trials Subcontract).